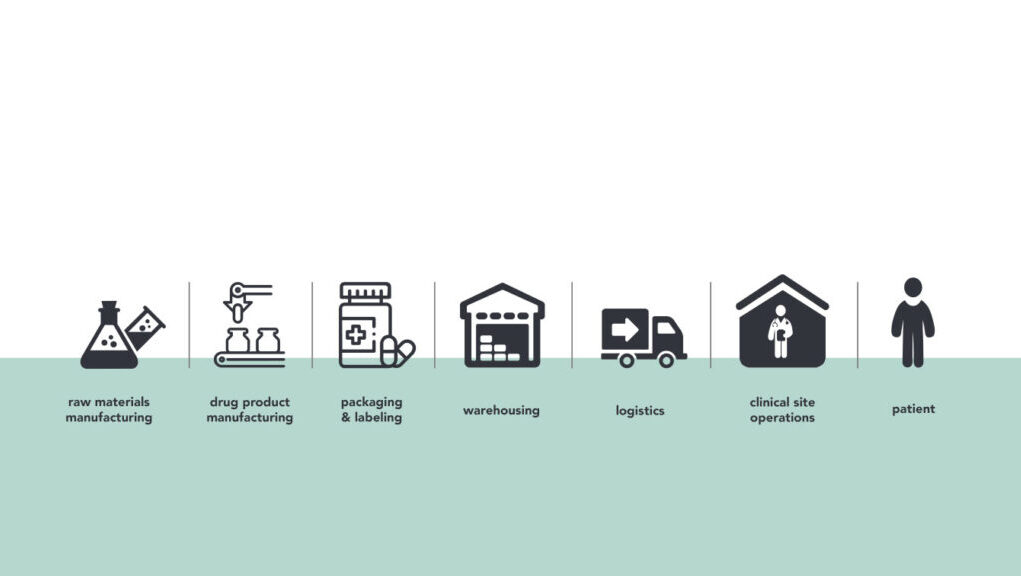
Clinical trial logistics planning involves numerous critical factors to ensure the smooth and efficient execution of medical research studies. From recruitment to data analysis, every stage requires meticulous attention to clinical trial logistics.
Ethical Considerations:
Ethical principles must guide every aspect of clinical trial logistics. This includes obtaining informed consent from participants, ensuring participant safety throughout the trial, and maintaining confidentiality of participant information. Ethical review boards must approve the trial protocol before recruitment begins.
Site Selection:
Selecting appropriate trial sites is crucial for the success of a clinical trial. Factors to consider include site infrastructure, expertise of investigators, patient population demographics, and availability of resources like laboratories and imaging facilities. Sites should also have experience in conducting similar trials.
Participant Recruitment and Retention:
Timely and effective recruitment of participants is often challenging in clinical trials. Factors such as eligibility criteria, geographical location, and patient demographics influence recruitment success. Strategies for recruitment should be carefully planned, including advertising, outreach to healthcare providers, and community engagement. Additionally, measures should be in place to enhance participant retention throughout the trial duration.
Logistical Planning for Investigational Products:
Proper management of investigational products (IPs) is essential to ensure their integrity and compliance with regulatory requirements. This includes procurement, storage, distribution, labeling, and accountability of IPs. Temperature-sensitive products require special handling and storage conditions.
Data Management:
Robust data management systems are necessary to collect, process, and analyze clinical trial data accurately and securely. Electronic data capture (EDC) systems are commonly used for data collection, with built-in features for data validation and quality control. Data management plans should outline procedures for data entry, verification, and storage, while ensuring data confidentiality and integrity.
Monitoring and Quality Assurance:
Regular monitoring of trial activities is essential to ensure compliance with the protocol, regulations, and quality standards. Monitoring visits assess data accuracy, participant safety, and protocol adherence. Quality assurance measures include audits, inspections, and training programs to maintain high standards throughout the trial.
Safety Reporting and Adverse Event Management:
Prompt reporting and management of adverse events (AEs) and serious adverse events (SAEs) are critical aspects of clinical trial logistics. Procedures should be in place for recording, assessing, reporting, and managing AEs according to regulatory requirements. Investigators must promptly communicate safety information to sponsors, ethics committees, and regulatory authorities.
Protocol Amendments:
Modifications to the trial protocol may be necessary to address emerging issues or optimize study procedures. Protocol amendments require careful planning and approval by regulatory authorities and ethics committees. Effective communication strategies are essential to ensure all stakeholders are aware of protocol changes and their implications.
Logistical Support for Study Visits:
Planning logistical support for study visits is essential to ensure smooth operations and participant satisfaction. This includes scheduling appointments, arranging transportation and accommodation for participants if necessary, and providing clear instructions for study procedures. Efficient clinic flow minimizes waiting times and enhances the overall participant experience.
Budget and Resource Management:
Developing a comprehensive budget is essential for allocating resources effectively throughout the trial. Budget considerations include personnel costs, site fees, participant reimbursements, equipment, and supplies. Continuous monitoring of expenditures and resource allocation ensures financial sustainability and adherence to budgetary constraints.
Communication and Collaboration:
Effective communication and collaboration among stakeholders are essential for successful clinical trial logistics planning. This includes regular meetings, clear documentation of responsibilities and expectations, and timely dissemination of information. Open channels of communication facilitate problem-solving and decision-making, ultimately contributing to the overall success of the trial.